with ≥1 prior line received 28-day cycles of daratumumab SC (same schedule as above) with Rd (R: 25
mg oral on Days 1-21; d: 40 mg IV/oral QW). Patients with TIE NDMM received nine 6-week cycles of
daratumumab SC (Cycle 1, QW; Cycles 2-9, Q3W Cycles 10+, Q4W in 28-day cycles) with VMP (V: 1.3
mg/m2 SC Cycle 1, twice weekly; Cycles 2-9, QW; M: 9 mg/m2; P: 60 mg/m2 oral on Days 1-4). Patients
were treated until disease progression or unacceptable toxicity. Primary endpoint: overall response
rate (ORR). Additional outcomes: very good partial response or better, complete response or better,
duration of response, minimal residual disease (MRD)-negative rate, daratumumab serum
concentrations, and safety.
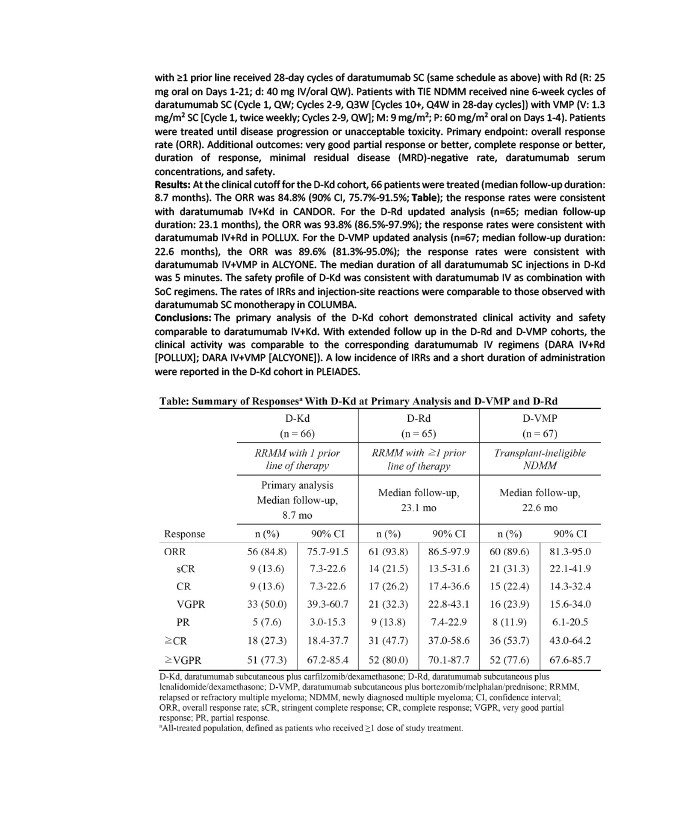
Results: At the clinical cutoff for the D-Kd cohort, 66 patients were treated (median follow-up duration:
8.7 months). The ORR was 84.8% (90% CI, 75.7%-91.5%; Table); the response rates were consistent
with daratumumab IV+Kd in CANDOR. For the D-Rd updated analysis (n=65; median follow-up
duration: 23.1 months), the ORR was 93.8% (86.5%-97.9%); the response rates were consistent with
daratumumab IV+Rd in POLLUX. For the D-VMP updated analysis (n=67; median follow-up duration:
22.6 months), the ORR was 89.6% (81.3%-95.0%); the response rates were consistent with
daratumumab IV+VMP in ALCYONE. The median duration of all daratumumab SC injections in D-Kd
was 5 minutes. The safety profile of D-Kd was consistent with daratumumab IV as combination with
SoC regimens. The rates of IRRs and injection-site reactions were comparable to those observed with
daratumumab SC monotherapy in COLUMBA.
Conclusions: The primary analysis of the D-Kd cohort demonstrated clinical activity and safety
comparable to daratumumab IV+Kd. With extended follow up in the D-Rd and D-VMP cohorts, the
clinical activity was comparable to the corresponding daratumumab IV regimens (DARA IV+Rd
POLLUX; DARA IV+VMP ALCYONE). A low incidence of IRRs and a short duration of administration
were reported in the D-Kd cohort in PLEIADES.