SCIENTIFIC PROGRAMME
SESSION I
HOW I TREAT
SMOLDERING MYELOMA
(SMM)
SESSION II
HOW I TREAT NEWLY
DIAGNOSED MULTIPLE
MYELOMA
SESSION III
FROM RISK
STRATIFICATION TO
RISK-BASED THERAPY?
DEBATE 1
SHOULD WE USE MRD
TESTING TO DETERMINE
THERAPY IN MULTIPLE
MYELOMA?
DEBATE 2
IS THERE A FUTURE ROLE
OF AUTOLOGOUS STEM
CELL TRANSPLANTATION?
SESSION IV
HOW I TREAT RELAPSED
MULTIPLE MYELOMA
DEBATE 3
SHOULD EVERY PATIENT
RECEIVE DARATUMUMAB
IN FIRST LINE?
ROUNDTABLE
MULTIPLE MYELOMA
FROM THE PERSPECTIVE
OF FDA/EMEA AND
FOUNDATIONS
SESSION V
YOU CAN’T BE IMMUNE
FOR IMMUNE THERAPY
ANYMORE
SESSION VI
OTHER PLASMA CELL
DYSCRASIAS
KEYNOTE LECTURES
THE FUTURE OF
MULTIPLE MYELOMA
SELECTED ABSTRACTS
FOR AN ORAL
PRESENTATION
ABSTRACTS SELECTED
AS POSTERS
DISCLOSURES
induction and consolidation, patients received R 25 mg orally (Days 1-14), V 1.3 mg/m2 subcutaneously
(Days 1, 4, 8, and 11), and d 40 mg QW every 21 days. Daratumumab 16 mg/kg was given intravenously
(Days 1, 8, and 15 of Cycles 1-4 and Day 1 of Cycles 5-6). During maintenance, patients received R 10
mg (15 mg in Cycles 10+, if tolerated) on Days 1-21 every 28 days ± daratumumab 16 mg/kg
intravenously Q8W (or Q4W per patient decision). The primary endpoint was sCR at the end of post-
ASCT consolidation per IMWG criteria. Key secondary endpoints included progression-free survival and
minimal residual disease (MRD)-negative rate (threshold, 10−5).
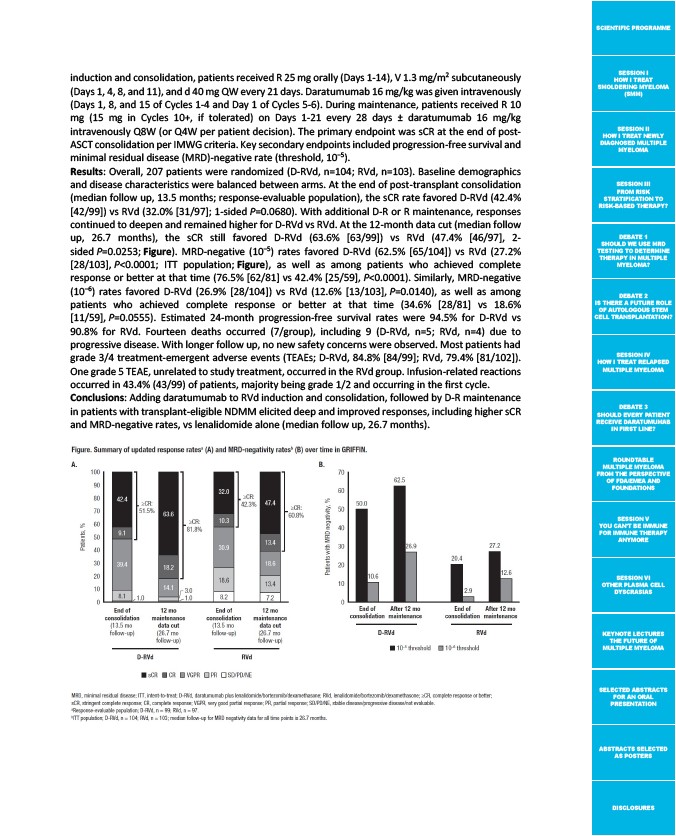
Results: Overall, 207 patients were randomized (D-RVd, n=104; RVd, n=103). Baseline demographics
and disease characteristics were balanced between arms. At the end of post-transplant consolidation
(median follow up, 13.5 months; response-evaluable population), the sCR rate favored D-RVd (42.4%
42/99) vs RVd (32.0% 31/97; 1-sided P=0.0680). With additional D-R or R maintenance, responses
continued to deepen and remained higher for D-RVd vs RVd. At the 12-month data cut (median follow
up, 26.7 months), the sCR still favored D-RVd (63.6% 63/99) vs RVd (47.4% 46/97, 2-
sided P=0.0253; Figure). MRD-negative (10‒5) rates favored D-RVd (62.5% 65/104) vs RVd (27.2%
28/103, P<0.0001; ITT population; Figure), as well as among patients who achieved complete
response or better at that time (76.5% 62/81 vs 42.4% 25/59, P<0.0001). Similarly, MRD-negative
(10‒6) rates favored D-RVd (26.9% 28/104) vs RVd (12.6% 13/103, P=0.0140), as well as among
patients who achieved complete response or better at that time (34.6% 28/81 vs 18.6%
11/59, P=0.0555). Estimated 24-month progression-free survival rates were 94.5% for D-RVd vs
90.8% for RVd. Fourteen deaths occurred (7/group), including 9 (D-RVd, n=5; RVd, n=4) due to
progressive disease. With longer follow up, no new safety concerns were observed. Most patients had
grade 3/4 treatment-emergent adverse events (TEAEs; D-RVd, 84.8% 84/99; RVd, 79.4% 81/102).
One grade 5 TEAE, unrelated to study treatment, occurred in the RVd group. Infusion-related reactions
occurred in 43.4% (43/99) of patients, majority being grade 1/2 and occurring in the first cycle.
Conclusions: Adding daratumumab to RVd induction and consolidation, followed by D-R maintenance
in patients with transplant-eligible NDMM elicited deep and improved responses, including higher sCR
and MRD-negative rates, vs lenalidomide alone (median follow up, 26.7 months).