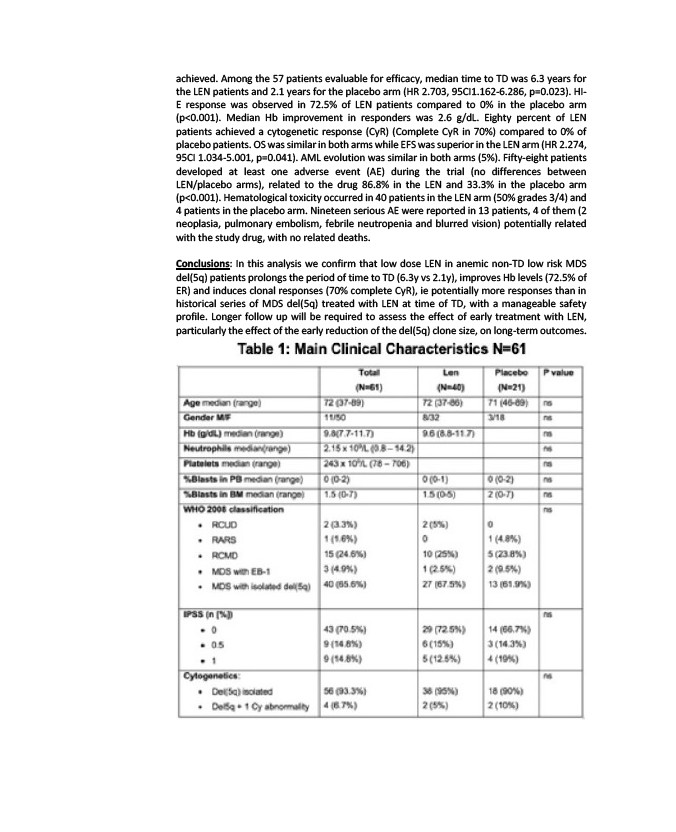
achieved. Among the 57 patients evaluable for efficacy, median time to TD was 6.3 years for
the LEN patients and 2.1 years for the placebo arm (HR 2.703, 95CI1.162-6.286, p=0.023). HI-E
response was observed in 72.5% of LEN patients compared to 0% in the placebo arm
(p<0.001). Median Hb improvement in responders was 2.6 g/dL. Eighty percent of LEN
patients achieved a cytogenetic response (CyR) (Complete CyR in 70%) compared to 0% of
placebo patients. OS was similar in both arms while EFS was superior in the LEN arm (HR 2.274,
95CI 1.034-5.001, p=0.041). AML evolution was similar in both arms (5%). Fifty-eight patients
developed at least one adverse event (AE) during the trial (no differences between
LEN/placebo arms), related to the drug 86.8% in the LEN and 33.3% in the placebo arm
(p<0.001). Hematological toxicity occurred in 40 patients in the LEN arm (50% grades 3/4) and
4 patients in the placebo arm. Nineteen serious AE were reported in 13 patients, 4 of them (2
neoplasia, pulmonary embolism, febrile neutropenia and blurred vision) potentially related
with the study drug, with no related deaths.
Conclusions: In this analysis we confirm that low dose LEN in anemic non-TD low risk MDS
del(5q) patients prolongs the period of time to TD (6.3y vs 2.1y), improves Hb levels (72.5% of
ER) and induces clonal responses (70% complete CyR), ie potentially more responses than in
historical series of MDS del(5q) treated with LEN at time of TD, with a manageable safety
profile. Longer follow up will be required to assess the effect of early treatment with LEN,
particularly the effect of the early reduction of the del(5q) clone size, on long-term outcomes.