STEPHEN(SCHUSTER((PHILADELPHIA)(
CAR?T(CELLS(
(
! Anti>CD19! chimeric! antigen! receptor! (or! “CAR”)! T! cell! therapies! have! changed! the!
prognosis!for!relapsed/refractory!large!B>cell!lymphomas,!whether!de(novo!or!arising!from!
histologic! transformation.! ! There! are! currently! two! US! FDA! and! EMA! approved! products!
available!for!commercial!use!for!this!indication:!axicabtagene(ciloleucel,!approved!in!the!US!
in!late!2017!based!on!the!ZUMA>1!trial1,2;!and,!tisagenlecleucel,!previously!approved!in!the!
US! in! 2017! for! relapsed/refractory! pediatric! and! young! adult! B>cell! precursor! acute!
lymphoblastic!leukemia!and!later!approved!in!the!US!in!early!2018!for!relapsed/refractory!
diffuse! large! B>cell! lymphomas! based! on! the! JULIET! trial3.! ! A! third! CAR>T! product,!
lisocabtagene( maraleucel,! is! expected! to! be! available! for! similar! indications! in! the! near!
future4.! ! All! products! either! have! or! will! have! indications! for! third>line! or! later! therapy! of!
relapsed!or!refractory!of!large!B>cell!lymphomas.!
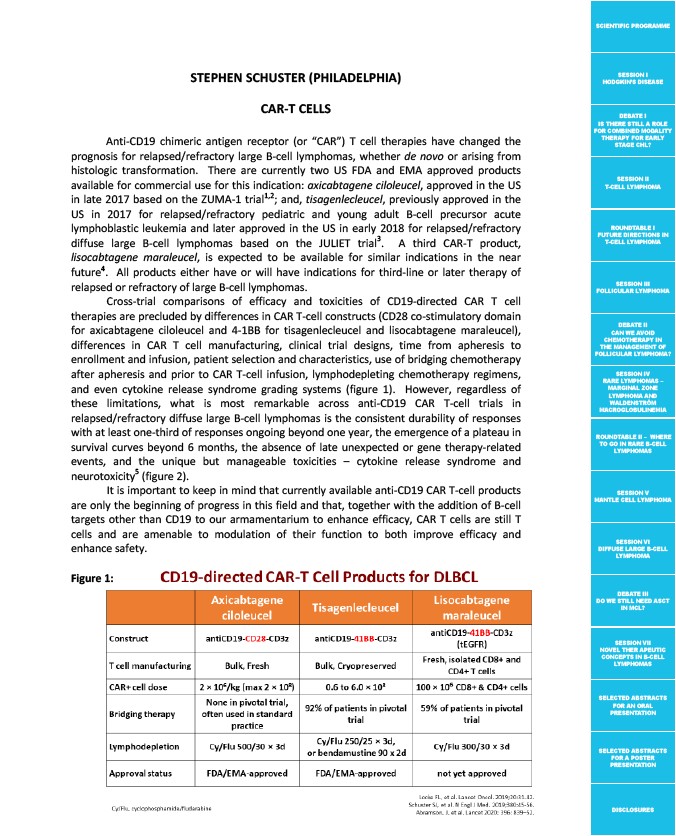
Cross>trial! comparisons! of! efficacy! and! toxicities! of! CD19>directed! CAR! T! cell!
therapies!are!precluded!by!differences!in!CAR!T>cell!constructs!(CD28!co>stimulatory!domain!
for! axicabtagene! ciloleucel! and! 4>1BB! for! tisagenlecleucel! and! lisocabtagene! maraleucel),!
differences! in! CAR! T! cell! manufacturing,! clinical! trial! designs,! time! from! apheresis! to!
enrollment!and!infusion,!patient!selection!and!characteristics,!use!of!bridging!chemotherapy!
after! apheresis! and! prior! to! CAR! T>cell! infusion,! lymphodepleting! chemotherapy! regimens,!
and! even! cytokine! release! syndrome! grading! systems! (figure! 1).! ! However,! regardless! of!
these! limitations,! what! is! most! remarkable! across! anti>CD19! CAR! T>cell! trials! in!
relapsed/refractory!diffuse!large!B>cell!lymphomas!is!the!consistent!durability!of!responses!
with!at!least!one>third!of!responses!ongoing!beyond!one!year,!the!emergence!of!a!plateau!in!
survival! curves! beyond! 6! months,! the! absence! of! late! unexpected! or! gene! therapy>related!
events,! and! the! unique! but! manageable! toxicities! –! cytokine! release! syndrome! and!
neurotoxicity5!(figure!2).!!!
It!is!important!to!keep!in!mind!that!currently!available!anti>CD19!CAR!T>cell!products!
are!only!the!beginning!of!progress!in!this!field!and!that,!together!with!the!addition!of!B>cell!
targets!other!than!CD19!to!our!armamentarium!to!enhance!efficacy,!CAR!T!cells!are!still!T!
cells! and! are! amenable! to! modulation! of! their! function! to! both! improve! efficacy! and!
enhance!safety.!
(
Figure(1:(
((((((((((((
SCIENTIFIC PROGRAMME
SESSION I
HODGKIN’S DISEASE
DEBATE I
IS THERE STILL A ROLE
FOR COMBINED MODALITY
THERAPY FOR EARLY
STAGE CHL?
SESSION II
T-CELL LYMPHOMA
ROUNDTABLE I
FUTURE DIRECTIONS IN
T-CELL LYMPHOMA
SESSION III
FOLLICULAR LYMPHOMA
DEBATE II
CAN WE AVOID
CHEMOTHERAPY IN
THE MANAGEMENT OF
FOLLICULAR LYMPHOMA?
SESSION IV
RARE LYMPHOMAS –
MARGINAL ZONE
LYMPHOMA AND
WALDENSTRÖM M
ACROGLOBULINEMIA
ROUNDTABLE II – WHERE
TO GO IN RARE B-CELL
LYMPHOMAS
SESSION V
MANTLE CELL LYMPHOMA
SESSION VI
DIFFUSE LARGE B-CELL
LYMPHOMA
DEBATE III
DO WE STILL NEED ASCT
IN MCL?
SESSION VII
NOVEL THER APEUTIC
CONCEPTS IN B-CELL
LYMPHOMAS
SELECTED ABSTRACTS
FOR AN ORAL
PRESENTATION
SELECTED ABSTRACTS
FOR A POSTER
PRESENTATION
DISCLOSURES