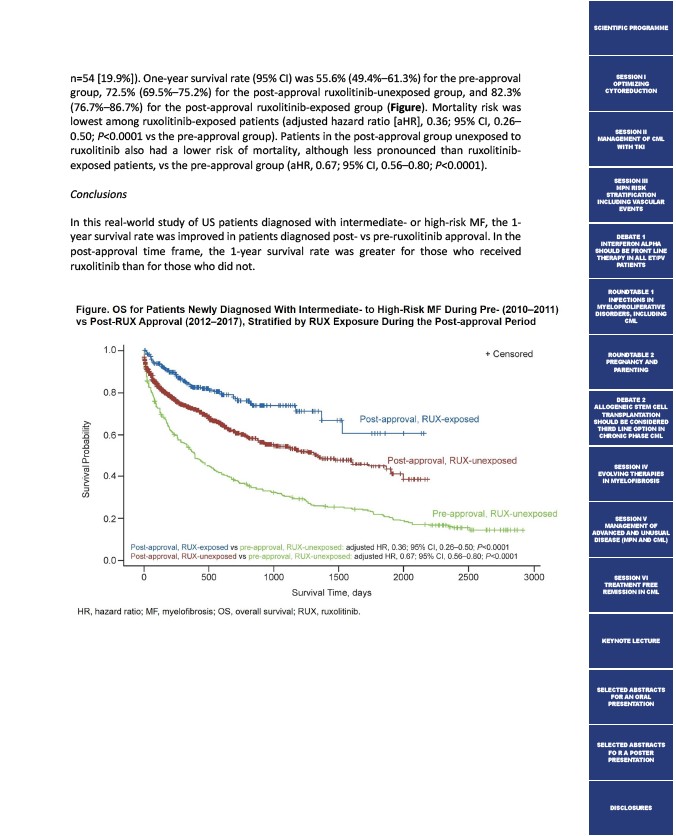
n=54 19.9%). One-year survival rate (95% CI) was 55.6% (49.4%–61.3%) for the pre-approval
group, 72.5% (69.5%–75.2%) for the post-approval ruxolitinib-unexposed group, and 82.3%
(76.7%–86.7%) for the post-approval ruxolitinib-exposed group (Figure). Mortality risk was
lowest among ruxolitinib-exposed patients (adjusted hazard ratio aHR, 0.36; 95% CI, 0.26–
0.50; P<0.0001 vs the pre-approval group). Patients in the post-approval group unexposed to
ruxolitinib also had a lower risk of mortality, although less pronounced than ruxolitinib-exposed
patients, vs the pre-approval group (aHR, 0.67; 95% CI, 0.56–0.80; P<0.0001).
Conclusions
In this real-world study of US patients diagnosed with intermediate- or high-risk MF, the 1-
year survival rate was improved in patients diagnosed post- vs pre-ruxolitinib approval. In the
post-approval time frame, the 1-year survival rate was greater for those who received
ruxolitinib than for those who did not.
SCIENTIFIC PROGRAMME
SESSION I
OPTIMIZING
CYTOREDUCTION
SESSION II
MANAGEMENT OF CML
WITH TKI
SESSION III
MPN RISK
STRATIFICATION
INCLUDING VASCULAR
EVENTS
DEBATE 1
INTERFERON ALPHA
SHOULD BE FRONT LINE
THERAPY IN ALL ET/PV
PATIENTS
ROUNDTABLE 1
INFECTIONS IN
MYELOPROLIFERATIVE
DISORDERS, INCLUDING
CML
ROUNDTABLE 2
PREGNANCY AND
PARENTING
DEBATE 2
ALLOGENEIC STEM CELL
TRANSPLANTATION
SHOULD BE CONSIDERED
THIRD LINE OPTION IN
CHRONIC PHASE CML
SESSION IV
EVOLVING THERAPIES
IN MYELOFIBROSIS
SESSION V
MANAGEMENT OF
ADVANCED AND UNUSUAL
DISEASE (MPN AND CML)
SESSION VI
TREATMENT FREE
REMISSION IN CML
KEYNOTE LECTURE
SELECTED ABSTRACTS
FOR AN ORAL
PRESENTATION
SELECTED ABSTRACTS
FO R A POSTER
PRESENTATION
DISCLOSURES